Immunotherapy
The Problem
Immunotherapy, a revolutionary cancer treatment, is transforming the fight against the disease. Yet, a significant hurdle remains: up to 60% of patients experience severe, unpredictable side effects. These organ-specific toxicities can be life-threatening and dramatically impact quality of life. The problem lies not just in the side effects themselves, but in their management.
Early detection and prompt intervention are crucial, but traditional healthcare systems, already stretched thin, struggle to keep pace with the rising tide of immunotherapy patients. This creates a critical gap in patient care. Delayed detection of side effects can lead to serious complications, and the burden on healthcare resources grows ever heavier. We need a new approach to managing immunotherapy’s side effects, a solution that prioritizes early detection and effective intervention.

Our Solution
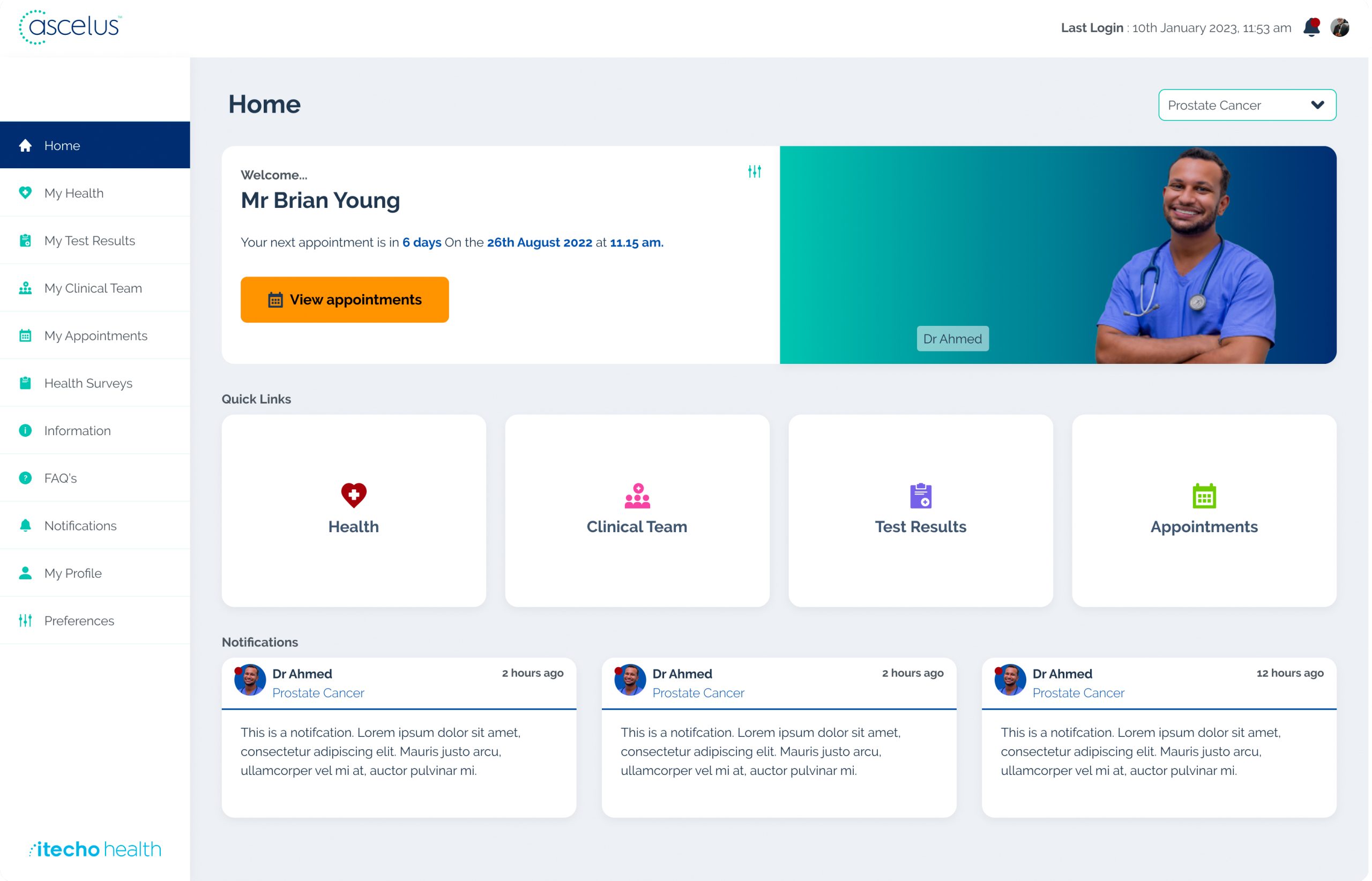
The answer lies in a paradigm shift. We need to embrace personalised care through digital patient pathways and remote monitoring for early detection and effective management. This shift will ensure patients reap the benefits of immunotherapy while minimising the burden on themselves and the healthcare system. This project will leverage the Ascelus platform to empower patients and transform care via:
Patient-centric design: Customisation of the Ascelus to address both patient and clinician needs in the management immunotherapy toxicity.
Real-time monitoring: Ascelus will provide early detection of side effects, enabling timely interventions and preventing complications.
Empowered patients: Real-time access to health data fosters patient engagement and active participation in managing their care.
Research, Innovate, Evaluate
We’re excited to announce our collaboration with the University of Sheffield on E-IMMUNE, a groundbreaking project funded by the Yorkshire Cancer Research Sheffield Pioneers Fund. This project aims to revolutionise immunotherapy toxicity management by empowering both patients and clinicians through a novel digital care pathway.
Working alongside academics, patients, and clinicians we will configure Ascelus to deliver a digital solution for Immunotherapy Toxicity Management.
In this research project, patients will be able to access their test results, report symptoms regularly, and conduct home-monitoring for vitals (blood pressure, pulse, oxygenation, glucose) directly through the Ascelus app on their smartphone or tablet. This will enable clinical teams can remotely monitor patient data and communicate directly through the app, reducing the need for unnecessary hospital visits.
Key aspects to this multidisciplinary research project include:
Platform optimisation (N=60): We’ll refine Ascelus based on feedback from a core group of patients.
Randomised stepped-wedge trial (N=502): Patients in Sheffield and Leeds will be assigned either digital care via Ascelus or usual care, with quality of life (QoL) as the primary outcome.
Late effects study (N=225): This study will assess the long-term impact of immunotherapy and Ascelus on patient well-being.
If successful, the programme will improve patient outcomes and cost-efficiency of NHS resource use in Yorkshire and establish a model for wider implementation across the NHS.
Collaborations / Partnerships
Sheffield Teaching Hospitals, NHS Trust, University of Sheffield, University of Leeds



This project is funded by the Yorkshire Cancer Research Sheffield Pioneers Fund



